Abstract
Background
Availability of molecularly targeted therapies has rapidly changed the treatment landscape for acute myeloid leukemia (AML). With these newer treatment choices, healthcare professionals (HCPs) must consider appropriate uses of molecular testing, treatment selection based on testing results, new sets of adverse events (AEs), and an increased need for patient education and shared decision-making (SDM). In this quality improvement (QI) initiative, we assessed barriers to evidence-based treatment planning for patients with AML in 4 community oncology systems and conducted team-based audit-feedback (AF) sessions within each system to facilitate HCP goal setting to mitigate identified barriers.
Methods
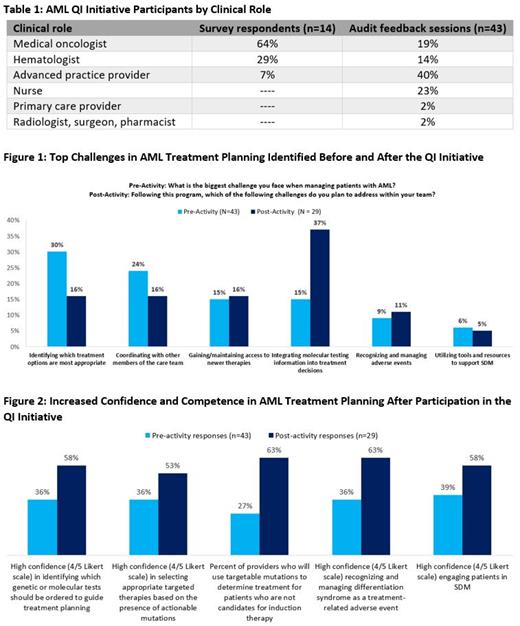
The QI initiative was conducted between Dec. 7, 2020 and Feb. 10, 2021. Initially, 14 hematology/oncology HCPs completed baseline team-based surveys to assess barriers in 4 community oncology systems (Table 1). To address identified gaps, 43 HCPs in the 4 systems participated in AF sessions. Action plans were developed by the clinical teams based on survey results (Table 1). Additional pre- and post-surveys completed before and after the AF sessions measured changes in participants' confidence and competence.
Results
On the pre-activity surveys, the most common challenges in AML management were: 1) identifying which treatment options are most appropriate (30%), 2) coordinating with other members of the care team (24%), 3) gaining/maintaining access to new therapies (15%), and 4) integrating molecular testing information into treatment decisions (15%) (Figure 1). After the 4 AF sessions, HCPs prioritized addressing these 4 challenges. In the baseline surveys, HCPs identified additional staff/resources (64%) and additional staff training/education as top resources needed to overcome these challenges.
HCPs identified additional challenges in the areas of AE management and patient centered care in the baseline surveys. Identified barriers in patient centered care, specifically, shared decision making (SDM), included not enough time to engage in SDM (57%) and patients' low health literacy (57%). After the 4 AF sessions, HCPs prioritized addressing tailoring treatment decisions to achieve patient goals (42%), coordinating follow-up visits with other care team members (32%), and engaging patients in shared decision-making (21%) as goals for improving patient-centered care.
Molecular testing emerged as a key challenge in AML treatment planning over the course of the QI initiative. Pre-activity, only 36% of HCPs expressed high confidence (4/5 Likert scale) in identifying which molecular tests should be ordered to guide treatment planning, and similarly only 36% were highly confident in selecting targeted therapies based on actionable mutations. HCPs also expressed dissatisfaction with ordering and timely receipt of molecular testing results in their systems. In post-activity surveys, over twice as many HCPs selected molecular testing as a challenge they were planning to address within their team (37% compared with 15% pre-activity), suggesting increased awareness of this barrier (Figure 1). Pre- to post-activity, HCPs who self-reported use of targetable mutations as an important factor in planning treatment for patients who are not candidates for induction therapy increased from 27% to 63%.
Participation in the QI initiative led to improvements in clinician confidence and competence in molecular testing, treatment selection based on molecular results, AE management, and use of SDM (Figure 2).
Conclusions
HCPs participating in this QI initiative identified barriers and potential areas for improvement in AML treatment planning, including barriers related to molecular testing, treatment selection and access, AE management, and patient-centered care. Participation in the AF sessions led to measurable improvements in HCP confidence and competence in key areas of AML management which may ultimately improve the quality of AML care in the community.
Study Sponsor Statement
The study reported in this abstract was funded by an independent educational grant from Genentech. The grantor had no role in the study design, execution, analysis, or reporting.
Blum: Abbvie: Honoraria; AmerisourceBergen: Honoraria; Forma Therapeutics: Research Funding; Leukemia and Lymphoma Society: Research Funding; Celyad Oncology: Research Funding; Nkarta: Research Funding; Xencor: Research Funding; Syndax: Honoraria. Fathi: Seattle Genetics: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Blueprint: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria; Agios: Consultancy, Honoraria, Research Funding; Servier: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria; Trillium: Consultancy, Honoraria; Kura: Consultancy, Honoraria; Foghorn: Consultancy, Honoraria; Kite: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria; Ipsen: Consultancy, Honoraria. Pollyea: Takeda: Honoraria; Syros: Consultancy, Honoraria; Syndax: Honoraria; Novartis: Consultancy, Honoraria; Kiadis: Honoraria; Aprea: Honoraria; Astellas: Honoraria; Bristol Myers Squibb: Honoraria; Celgene: Honoraria; Foghorn: Honoraria; Genentech: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Jazz: Honoraria; Karyopharm: Consultancy, Honoraria; Amgen: Honoraria; AbbVie: Consultancy, Honoraria, Research Funding; Teva: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal